When scientists study radioactive materials like Uranium-235, one important thing they look at is how fast it breaks down, or decays, over time. This process is measured using two key things:
- Half-life: how long it takes for half of the material to decay.
- Decay constant (λ): a number that shows how quickly the substance decays.
If you know the half-life of a substance, you can calculate the decay constant easily. Let’s walk through it step-by-step using Uranium-235 as an example.
You can watch this Video to Get a clearer understanding of what I’m talking about, or you can chose to check out the steps on howI solved it below.
What is the Half-Life of Uranium-235?
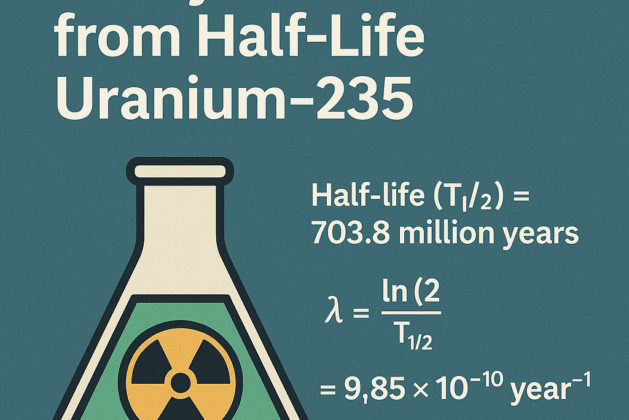
Uranium-235 is a radioactive isotope used in nuclear reactors and weapons. Its half-life is:
🧪 Half-life (T½) = 703.8 million years
That’s 703,800,000 years in full numbers.
Formula to Use
The formula to calculate the decay constant (λ) is: λ=ln(2)T1/2\lambda = \frac{\ln(2)}{T_{1/2}}λ=T1/2ln(2)
Where:
- λ\lambdaλ is the decay constant
- ln(2)\ln(2)ln(2) is the natural logarithm of 2 (which is approximately 0.693)
- T1/2T_{1/2}T1/2 is the half-life
Step-by-Step Calculation
Let’s now plug in the values.
Step 1: Write the formula
λ=ln(2)T1/2\lambda = \frac{\ln(2)}{T_{1/2}}λ=T1/2ln(2) λ=0.693703,800,000 years\lambda = \frac{0.693}{703,800,000 \text{ years}}λ=703,800,000 years0.693
Step 2: Do the division
λ=9.846×10−10 per year\lambda = 9.846 \times 10^{-10} \text{ per year}λ=9.846×10−10 per year
✅ This means the decay constant of Uranium-235 is approximately: 9.85×10−10 year−1\boxed{9.85 \times 10^{-10} \text{ year}^{-1}}9.85×10−10 year−1
What Does This Mean?
This tiny number tells us how slowly Uranium-235 decays. Since the decay constant is small, it means the substance breaks down very slowly — which is why Uranium-235 can remain radioactive for billions of years.
Why is This Useful?
Knowing the decay constant helps scientists:
- Predict how much Uranium-235 will remain after a long time
- Estimate the age of rocks and fossils (radioactive dating)
- Manage nuclear waste safely
Quick Summary
| Quantity | Value |
|---|---|
| Half-life of U-235 | 703.8 million years |
| Natural log of 2 | 0.693 |
| Decay Constant (λ) | 9.85 × 10⁻¹⁰ year⁻ |
If you ever need to calculate how fast something radioactive is decaying, just remember the simple formula: λ=0.693Half-life\lambda = \frac{0.693}{\text{Half-life}}λ=Half-life0.693
And with that, you’ve just learned how to find the decay constant from the half-life


Leave a comment